Microwave-mediated stereocontrolled annulations of diazo(aryl)methyl(diaryl)phosphine oxides with pyridinium 1,4-zwitterionic thiolates†
Abstract
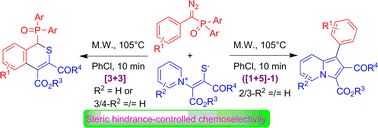
Chemoselective annulations of phosphoryl carbenes generated from diazo(aryl)methyl(diaryl)phosphine oxides with pyridinium 1,4-zwitterionic thiolates were performed under microwave irradiation, affording 1-diarylphosphoryl-1H-benzo[c]thiopyran derivatives via [3+3] annulation and indolizine derivatives via ([1+5]-1) annulation with P-Cope elimination as the key step. The annuloselectivity was controlled by the steric hindrance of pyridiniums in pyridinium 1,4-zwitterionic thiolates.


 Please wait while we load your content...
Please wait while we load your content...