Enantioselective synthesis of (R)-2-cubylglycine including unprecedented rhodium mediated C–H insertion of cubane†
Abstract
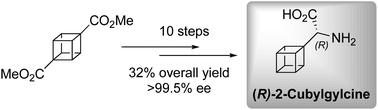
The first enantioselective synthesis of (R)-2-cubylglycine, an analogue of (R)-2-phenylglycine in which the phenyl ring has been replaced by cubane, is disclosed. The key step was a telescoped Strecker reaction using (S)-2-amino-2-phenylethanol as a chiral auxiliary. Exploration of an alternative synthetic approach resulted in unprecedented cubane C–H insertion.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...