Structure and chemical stability in perovskite–polymer hybrid photovoltaic materials†
Abstract
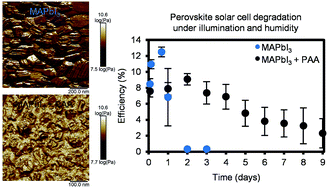
Adding polymers to methylammonium lead iodide perovskite solar cell active layers has been previously shown to increase their chemical stability, but stabilization mechanisms in these hybrid materials are poorly understood. We report here on a structural and spectroscopic analysis in a number of perovskite–polymer hybrid materials and compare their stability. We observed that perovskite crystallite sizes decrease with the addition of polymers (polyethylene glycol, polyethyleneimine, poly(acrylic acid) and polyvinylpyrrolidone), and through the use of nanomechanical AFM showed phase contrast in the perovskite–polymer mixture. NMR and single-crystal growth experiments reveal that acid–base interactions, as well as facet-dependent interfacial interactions between perovskite and polymer, contribute to differences in stabilities of these hybrid materials. The polymers investigated tend to suppress the formation of a hydrate crystal phase that accelerates the degradation reaction, and we report that adding poly(acrylic acid) increases significantly the stability of perovskite films under humid air and ambient illumination. Under these controlled degradation conditions, perovskite–poly(acrylic acid) hybrid solar cells maintain stable efficiency for the first 3 days and then slowly degrade over the next 6 days under humid air and illumination, whereas control perovskite solar cells degrade entirely within the first 2 days. These results highlight the importance of choosing suitable functional groups in the polymer phase of perovskite hybrid solar cells to prolong their device lifetime.


 Please wait while we load your content...
Please wait while we load your content...