Electrocatalysis of hydrogen production by active site analogues of the iron hydrogenase enzyme: structure/function relationships†
Abstract
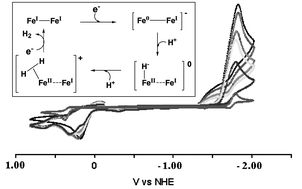
A series of binuclear FeIFeI complexes, (μ-SEt)2[Fe(CO)2L]2 (L = CO (1), PMe3 (1-P)), (μ-SRS)[Fe(CO)2L]2 (R = CH2CH2 (μ-edt): L = CO (2), PMe3 (2-P); R = CH2CH2CH2(μ-pdt): L = CO (3), PMe3 (3-P); and R = o-CH2C6H4CH2 (μ-o-xyldt): L = CO (4), PMe3 (4-P)), that serve as structural models for the active site of Fe-hydrogenase are shown to be electrocatalysts for H2 production in the presence of acetic acid in acetonitrile. The redox levels for H2 production were established by spectroelectrochemistry to be Fe0Fe0 for the all-CO complexes and FeIFe0 for the PMe3-substituted derivatives. As electrocatalysts, the PMe3 derivatives are more stable and more sensitive to acid concentration than the all-CO complexes. The electrocatalysis is initiated by electrochemical reduction of these diiron complexes, which subsequently, under weak acid conditions, undergo protonation of the reduced iron center to produce H2. An (η2-H2)FeII–Fe0/I intermediate is suggested and probable electrochemical mechanisms are discussed.
- This article is part of the themed collection: Celebrating 50 years of Dalton Transactions: Our Top 50

 Please wait while we load your content...
Please wait while we load your content...