Ni2P nanoflakes for the high-performing urea oxidation reaction: linking active sites to a UOR mechanism†
Abstract
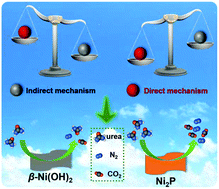
Urea electrolysis is regarded as an effective method for addressing both energy and environment issues. Herein, we successfully synthesized Ni2P nanoflakes for catalyzing the urea oxidation reaction (UOR). Due to the higher electrical conductivity as well as the prevailing tendency in triggering the UOR via a direct electro-oxidation mechanism, Ni2P nanoflakes exhibit comparable UOR activity (1.33 V vs. RHE for onset-potential, and 95.47 mA·cm−2 at 1.6 V vs. RHE) to the most active state-of-the-art catalysts, rendering them an effective alternative to precious metals such as Pt and Rh. The accelerated proton-coupled electron transfer (PCET) process caused by PO43− facilitates the in situ generation of NiOOH; thus, the UOR process is initiated at a lower onset-potential on Ni2P nanoflakes than on β-Ni(OH)2 nanoflakes. The in situ generated NiOOH instead of the Ni2P phase in Ni2P nanoflakes functions as an active site during the UOR process, while both NiOOH and the Ni2P phase serve as active sites in the OER process. This work provides insights into the understanding of the UOR mechanism and opens a new avenue to design low-cost Ni-based phosphide UOR catalysts.
- This article is part of the themed collection: Nanoscale Horizons, Nanoscale, and ChemComm: Nanocatalysis


 Please wait while we load your content...
Please wait while we load your content...