Sustainable preparation of photoactive indole-fused tetracyclic molecules: a new class of organophotocatalysts†
Abstract
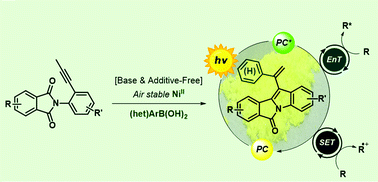
Efficient and sustainable preparation of photoactive indole-fused tetracyclic molecules and their application as organophotocatalysts have been developed. Additive-free and air stable nickel catalysis of readily prepared phthalimide derivatives in an alcoholic solvent provided a new class of bench-stable tetracyclic functional molecules in excellent yield without complicated purification steps. Extensive investigations of the photophysical and electrochemical properties of the new molecules, as well as TD-DFT studies, revealed their potential to act as both energy- and electron transfer organophotocatalysts, which was further demonstrated through a series of sustainable photochemical applications. Moreover, the molecules with high quantum yields and strong solid-state emissions show high potential to be green alternatives to metal-based materials.


 Please wait while we load your content...
Please wait while we load your content...