A relaxor ferroelectric polymer with an ultrahigh dielectric constant largely promotes the dissociation of lithium salts to achieve high ionic conductivity†
Abstract
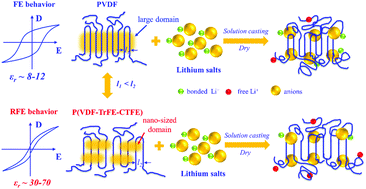
The extremely low room-temperature ionic conductivity of solid-state polymer electrolytes (SPEs) ranging from 10−7 to 10−5 S cm−1 seriously restricts their practical application in solid-state lithium metal batteries (LMBs). Herein, a unique relaxor ferroelectric (RFE) polymer of poly(vinylidene fluoride-co-trifluoroethylene-co-chlorotrifluoroethylene) [P(VDF-TrFE-CTFE)] is first investigated as a matrix of SPEs. We find that the P(VDF-TrFE-CTFE) with an ultrahigh dielectric constant (εr) of 44 presents a stronger solvation ability towards lithium ions, which promotes the dissociation of LiN(SO2CF3)2 to form more free charge carriers and enhances their mobility compared to the conventional PVDF with a low εr of 9. The P(VDF-TrFE-CTFE) based SPEs show a much higher ionic conductivity of 3.10 × 10−4 S cm−1 at 25 °C and lower activation energy (0.26 eV) than PVDF based SPEs (1.77 × 10−5 S cm−1 and 0.49 eV). The PVDF blended with the P(VDF-TrFE-CTFE) or dielectric fillers such as BaTiO3 further confirm that the hybrid electrolytes with a larger εr show a higher ionic conductivity. In addition, very tight interfaces of P(VDF-TrFE-CTFE) based SPEs with both the cathode and Li metal anode are constructed to ensure a stable interfacial resistance during cycling. The LiFePO4/Li and LiNi0.8Co0.1Mo0.1O2/Li batteries using P(VDF-TrFE-CTFE) based SPEs present a stable cycling performance at 25 °C. This work proposes a new strategy and opens a new research area to construct SPEs with high ionic conductivity by greatly increasing the εr of polymers.


 Please wait while we load your content...
Please wait while we load your content...