Cu2CoGeS4 nanocrystals for high performance aqueous polysulfide/iodide redox flow batteries: enhanced selectively towards the electrocatalytic conversion of polysulfides†
Abstract
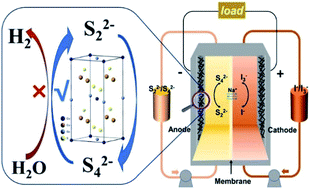
Aqueous polysulfide/iodide redox flow batteries (RFBs) are an attractive candidate for scalable energy storage with highly soluble active materials, which show great potential to reduce RFB cost. However, their energy efficiency is usually limited by poor kinetics reversibility and electrochemical activity of the redox reaction of polysulfide couples on the graphite felt electrode, which hinder its further practical applications. Here, Cu2XGeS4 (X = Fe, Co, Ni, Cd, Mn) nanocrystals are successfully synthesized through the hot injection method, and then uniformly coated onto the surface of graphite felt as electrodes. These electrodes can successfully suppress the water splitting side reactions, especially the hydrogen evolution reaction. The ones with Cu2CoGeS4 nanocrystals can significantly boost electrocatalytic activities of S2−/Sx2− redox reactions for the proper adsorbability of polysulfide, which inturn improve the charge transfer process and selectively facilitate the electro-conversion reactions of polysulfides. Furthermore, the polysulfide/iodide flow battery with GF–Cu2CoGeS4 generates a high energy efficiency of 77.2% at 20 mA cm−2, a power density of 40.7 mW cm−2 and a stable energy efficiency retention of 92% after approximately 250 continuous cycles.


 Please wait while we load your content...
Please wait while we load your content...