A metal-free tandem approach to prepare structurally diverse N-heterocycles: synthesis of 1,2,4-oxadiazoles and pyrimidinones†
Abstract
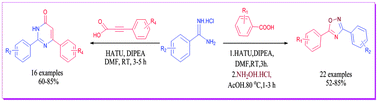
A metal-free one-pot approach to the diversity oriented synthesis of N-heterocycles, 1,2,4-oxadiazoles and 2,6 disubstituted pyrimidin-4-ones is described via carboxamidation of amidines with aryl carboxylic acids and aryl propargylic acids. The reactions occur at room temperature forming N-acylamidines which undergo tandem nucleophilic addition–deamination–intramolecular cyclisation to give the corresponding heterocyclic compounds in good to excellent yields. This one pot approach has led to the successful synthesis of the drug lead molecule, ataluren, 3-(5-(2-fluorophenyl)-1,2,4-oxadiazol-3-yl) benzoic acid in two steps.

 Please wait while we load your content...
Please wait while we load your content...