A substrate-dependent mechanism for nickel-catalyzed N-allylation with allylic alcohols: nucleophilic attack vs. reductive elimination†
Abstract
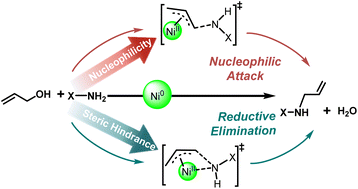
Transition metal catalyzed amination of allylic alcohols via π-allylmetal is generally accepted to undergo a nucleophilic attack mechanism. However, we herein found the possibility of competition between reductive elimination and nucleophilic attack, which is dependent on substrates. A DFT study on the mechanism of nickel-catalyzed N-allylation with allylic alcohols using different amides/amines reveals that substrates with stronger nucleophilicity prefer the conventional nucleophilic attack mechanism. In contrast, substrates with lower nucleophilicity and larger steric hindrance may prefer the reductive elimination mechanism instead. The presented mechanistic information should be helpful for the development of transition metal catalyzed allylation chemistry.


 Please wait while we load your content...
Please wait while we load your content...