Branching effect for aggregation-induced emission in fluorophores containing imine and triphenylamine structures†
Abstract
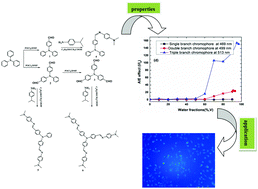
Three new chromophores incorporating donor–π–donor–π–donor structural motifs and single, double and triple branched 4-(N,N′-dimethylamine) phenyl groups linked to triphenylamine through an imine π-bridge were synthesized. The photoluminescence properties of three the chromophores were studied in the solution and aggregated states. It was demonstrated that the aggregation-induced emission (AIE) effect was enhanced when the branch number was increased. The AIE mechanism for the three compounds was explained by the molecule stacking mode in the crystal structures. The propeller shaped non-planar molecular configuration of tri-branched triphenylamine inhibited face to face π–π stacking, which provided favorable 3D structures to restrict intramolecular rotation and benefited AIE effects. TEM, DLS and fluorescent microscopy imaging revealed the nano-sized aggregate state of the THF–H2O mixture. Fluorescence imaging experiments of the tri-branched chromophore in living A549 cells proved its potential for real-world applications.

 Please wait while we load your content...
Please wait while we load your content...