Binary NiCoO2-modified graphite felt as an advanced positive electrode for vanadium redox flow batteries†
Abstract
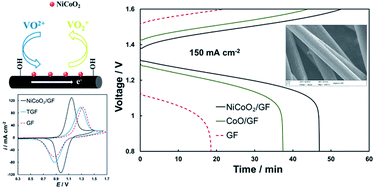
To address the challenges of the poor electrochemical performance of graphite felt electrodes in vanadium redox flow batteries (VRFBs), binary nickel cobalt oxide (NiCoO2) is developed as a novel candidate for the positive electrode via a facile hydrothermal and calcination approach. The graphite felt modified with NiCoO2 exhibits excellent electrochemical activity and reversibility in the VO2+/VO2+ redox reactions resulting from the enhanced electrocatalytic activity of binary NiCoO2, compared with pristine graphite felt and single metal oxide CoO-modified graphite felt. The flow cell employing a NiCoO2-modified graphite felt electrode displays a voltage efficiency of 81.2% at a high current density of 150 mA cm−2, which is higher than that of 66.9% and 77.5% obtained from pristine graphite felt and CoO-modified graphite felt electrodes, respectively. In particular, the discharge capacity of the flow cell is significantly enhanced, increasing from 185.4 mAh for graphite felt and 374.2 mAh for CoO-modified graphite felt to 469.4 mAh for NiCoO2-modified graphite felt. No obvious efficiency decay is observed after 50 charge–discharge cycles confirming the stability of NiCoO2 deposition on graphite felt. Overall, the results demonstrate that the electrochemical performance of graphite felt can be further enhanced via introducing a binary metal oxide exhibiting higher conductivity and electrocatalytic activity compared with single metal oxides, providing guidelines for future research in VRFB studies.


 Please wait while we load your content...
Please wait while we load your content...