Fabrication of SiO2@COF5 microspheres and their application in high performance liquid chromatography†
Abstract
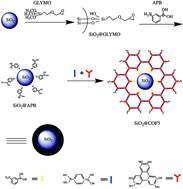
The structural regularity, atomically precise design, high surface area and adsorption affinity make covalent-organic frameworks (COFs) attractive as novel stationary phases in chromatography. In this paper, an in situ growth strategy was developed to synthesize a COF–silica composite (SiO2@COF5 microspheres) by modifying COF5 onto silica particles (5 μm) as the stationary phase for HPLC separation. The COF5 shell was constructed from benzene-1,4-diboric acid and 2,3,6,7,10,11-hexahydroxytriphenylene. The in situ growth of the COF5 shell not only on the particle surface but also inside the pore of the SiO2 particles overcomes the shortcomings of the irregular morphology of the COF and high back pressure from the sub-micrometer size of the COF, facilitating its application in HPLC. The enthalpy change and entropy change of analytes (alkyl benzenes and polycyclic aromatic hydrocarbons) during the separation with SiO2@COF5 as the stationary phase were first measured by varying the column temperature to investigate the retention mechanism. The enthalpy change and entropy change were found different from commercial C18 columns, suggesting the contribution of the three-dimensional structure of SiO2@COF5 microspheres. The SiO2@COF5 packed column (150 mm × 2.1 mm i.d.) showed excellent selectivity for alkyl benzenes, polycyclic aromatic hydrocarbons, anilines, acetophenones, benzaldehydes and isomers of hydroxyacetophenone. The SiO2@COF5 packed column also showed good repeatability with relative standard deviations of retention time and peak height below 5%.


 Please wait while we load your content...
Please wait while we load your content...