Osteoimmunomodulation, osseointegration, and in vivo mechanical integrity of pure Mg coated with HA nanorod/pore-sealed MgO bilayer†
Abstract
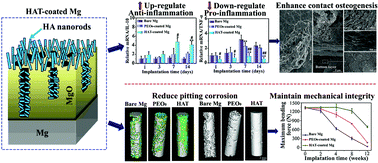
Fast degradation of Mg-based implants results in the loss of mechanical integrity and poor osseointegration. Herein, a bilayer-structured coating (termed as HAT), comprising an outer layer of hydroxyapatite (HA) nanorods and an inner layer of pores-sealed MgO with HA/Mg(OH)2, was formed on Mg using plasma electrolytic oxidation and hydrothermal treatment. Osteoimmunomodulation, osseointegration, mechanical integrity, and bone–implant interfacial structure evolution of the HAT-coated Mg were investigated by implantation in rabbit femora, together with Mg coated with plasma electrolytic oxidized porous MgO (termed as PEO0) and bare Mg. As compared to PEO0-coated and bare Mg, HAT-coated Mg greatly downregulated pro-inflammatory TNF-α and IL-1β, upregulated anti-inflammatory IL-10, and suppressed osteoclastogenesis, modulating the surrounding microenvironment toward favoring the recruitment of osteogenetic cells. Moreover, HAT-coated Mg accelerated bone sialoprotein and osteopontin secretion of osteogenetic cells and their mineralization to form a cement line matrix. It also promoted the differentiation of osteogenetic cells, secretion of collagen overlying on the cement line matrix, inducing an earlier and more pronounced bone matrix formation. The cement line matrix wrapped the HA nanorods and filled the interrod spaces of the HAT coating, forming strong interdigitation at the bone–coating interface, and therefore, yielding enhanced osseointegration by means of contact osteogenesis. Due to the considerably reduced corrosion of Mg by the pores-sealed bilayer structure of HAT coating, HAT-coated Mg maintained the mechanical integrity for a longer duration than PEO0-coated and bare Mg. It is clarified that the degradation of MgO and HA, rather than delamination, was the vanishing mode of PEO0 and HAT coatings during long-term implantation, avoiding osteolysis induced by the delamination-generated particles.


 Please wait while we load your content...
Please wait while we load your content...