Visible detection of copper ions using a fluorescent probe based on red carbon dots and zirconium metal–organic frameworks†
Abstract
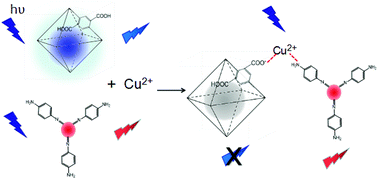
A highly selective and sensitive ratiometric fluorescent probe for copper ions (Cu2+) in aqueous solution based on a blue zirconium metal–organic framework [UiO-66-(COOH)2] with red bandgap fluorescent carbon quantum dots (R-BF-CQDs) is developed. UiO-66-(COOH)2 displays broad ligand-centered (LC) emission and R-BF-CQDs shows red emission. Interestingly, the remaining –NH2 at the surface of the prepared R-BF-CQDs can coordinate Cu2+ efficiently and produce a strong visible absorption, which overlaps the emission of UiO-66-(COOH)2. There is also a strong coordination capacity of the Cu2+ ions with R-BF-CQDs and UiO-66-(COOH)2, which allows the energy donor and the energy receptor to be closer together. Both of these two conditions provide the possibility of spectral energy transfer. In practice, the LC emission of UiO-66-(COOH)2 becomes weaker with increasing concentration of Cu2+, whereas the red fluorescence of R-BF-CQDs increases slightly.


 Please wait while we load your content...
Please wait while we load your content...