Constructing oxygen vacancies in anatase TiO2: enhancing acetylene semi-hydrogenation performance through H2 heterolytic cleavage†
Abstract
Oxygen vacancies (OV) constitute significant defects in TiO2 and various other metal oxides, profoundly influencing heterogeneous catalytic reactions. In this investigation, anatase TiO2 samples with different OV concentrations were synthesized by adjusting both the atmosphere and temperature. Characterization results reveal a gradual increase in relative OV content with higher H2 reduction temperatures, contrasting with observations made under an air atmosphere. Through acetylene semi-hydrogenation reactions, it was validated that elevated OV content promotes the reaction activity of acetylene hydrogenation. Kinetic studies have shown that the acetylene reaction rate is positively correlated with the OV content. Density functional theory (DFT) calculations demonstrated that hydrogen undergoes homolytic cleavage on TiO2 surfaces devoid of OV, whereas surfaces containing OV experience heterolytic cleavage, resulting in a significant decrease in hydrogen activation energy and thereby enhancing reaction activity. Furthermore, OV demonstrate higher adsorption affinity for acetylene, thereby facilitating its hydrogenation. On OV-rich surfaces, the rate-determining step for acetylene semi-hydrogenation is the initial hydrogenation 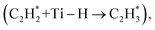 which requires less energy (0.14 eV). Conversely, on OV-lacking surfaces, the rate-determining step shifts to the second hydrogenation
which requires less energy (0.14 eV). Conversely, on OV-lacking surfaces, the rate-determining step shifts to the second hydrogenation 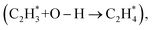 which needs to overcome an energy of 5.03 eV. Consequently, acetylene can be efficiently hydrogenated to ethylene on TiO2 surfaces with OV. Based on our experimental findings, TiO2 samples obtained through hydrogen reduction at 200 °C demonstrate optimal performance for acetylene semi-hydrogenation, achieving a 100% conversion rate and 95% ethylene selectivity under the employed reaction conditions.
which needs to overcome an energy of 5.03 eV. Consequently, acetylene can be efficiently hydrogenated to ethylene on TiO2 surfaces with OV. Based on our experimental findings, TiO2 samples obtained through hydrogen reduction at 200 °C demonstrate optimal performance for acetylene semi-hydrogenation, achieving a 100% conversion rate and 95% ethylene selectivity under the employed reaction conditions.



 Please wait while we load your content...
Please wait while we load your content...