Two for one: propylene carbonate co-solvent for high performance aqueous zinc-ion batteries – remedies for persistent issues at both electrodes†
Abstract
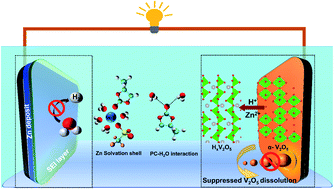
Aqueous rechargeable zinc-ion batteries are attractive for large-scale energy storage applications. However, challenges such as the dendritic growth of zinc, structural degradation and dissolution of oxide cathodes, and poor coulombic efficiencies (CEs) limit their scope. While organic co-solvents and additives in low concentration Zn2+-ion electrolytes have had some success in redressing these issues, their efficacy is tempered by them being required in large amounts which reduces key electrolyte features such as electrical conductivity. Herein, we report a significant enhancement in battery characteristics upon the introduction of the highly polar propylene carbonate (PC) which imparts its dual-functionality – it suppresses water-derived parasitic reactions occurring at both the Zn anode and the V2O5 cathode. Upon a small addition of PC (≤20 wt%) in 1 M zinc triflate electrolyte, water molecules solvating Zn2+-ions are shown to be partly replaced by the carbonate and the triflate anions, as evidenced by spectroscopic and molecular dynamics simulation studies. Subsequently, the PC and triflate anion get electrochemically reduced to form a ZnF2-rich solid-electrolyte interphase, resulting in a stable zinc plating/stripping process in Zn|Cu cells for 700 cycles with CEs of ∼99.3%. The highly basic carbonyl oxygen of PC is shown to strongly interact with water molecules, which in turn suppresses the dissolution of the cathode, enabling exceptional capacities of ∼300 mA h g−1 over 900 cycles at 1 A g−1.


 Please wait while we load your content...
Please wait while we load your content...