Ultrathin g-C3N4 nanosheets with hexagonal CuS nanoplates as a novel composite photocatalyst under solar light irradiation for H2 production†
Abstract
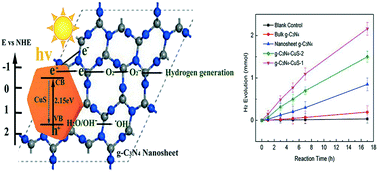
Using photocatalysts and solar energy to split water into hydrogen is an ideal energy source, hereto, a novel hybrid multifunctional g-C3N4–CuS nanocomposite photocatalyst was synthesized for H2 evolution by integrating g-C3N4 nanosheets with hexagonal CuS nanoplates via a facile hydrothermal method at room temperature. The ultrathin large surface area g-C3N4 nanosheets were prepared by a “green” liquid exfoliation route from bulk g-C3N4 in isopropanol. Different nanostructural g-C3N4 materials were compared and the results confirmed that the g-C3N4 nanosheet had better hydrogen evolution than the bulk material under solar irradiation due to its larger surface area and higher light absorption efficiency. Furthermore, given the improved charge separation efficiency, the novel nanocomposite g-C3N4–CuS-1 demonstrated a much better hydrogen evolution rate (126.5 μmol h−1) than the pure g-C3N4 nanosheet under solar light. Its performance is also superior to that of other semiconductor modified g-C3N4 materials, making it a promising photocatalyst for future hydrogen generation application. The optical properties, crystal phase and morphology of the obtained composites were characterized systematically using various analytical methods. It was concluded that O2˙− was the dominant radical for hydrogen evolution through photoluminescence (PL) and electron paramagnetic resonance (EPR) analysis.


 Please wait while we load your content...
Please wait while we load your content...