Bell-shaped RuFe/Ni5P4 for efficient urea-assisted electrolytic hydrogen production†
Abstract
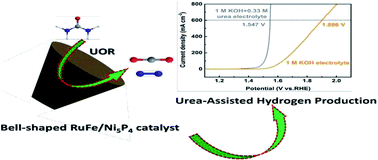
Urea-assisted electrolytic hydrogen production requires much lower theoretical cell voltage than the conventional water splitting technology (Eθ = 0.37 V vs. 1.23 V), serving as a promising alternative for concepts of “clean energy” and “cleaning pollutant”. However, screening highly robust catalysts with industrial-level current density (>200 mA cm−2) is still challenging. To overcome this challenge, bell-shaped RuFe/Ni5P4 catalysts grown on nickel foam (NF) were developed via dual doping of Fe and Ru and a facile phosphidation process. The unique bell-shaped structure significantly contributes to the improved contact with the electrolyte, leading to the promoted mass transfer as well as the efficient release of CO2 and N2 bubbles generated during the urea oxidation reaction (UOR). Moreover, the strong electronic interaction between Ru and Ni5P4 ensures high UOR activity with a high current density of 600 mA cm−2 at 1.45 V (vs. RHE). Moreover, an assembled Ru/NF|KOH, CO(NH2)2|RuFe/Ni5P4 electrolytic cell demonstrated a highly efficient hydrogen production rate in order to reach a current density of 600 mA cm−2 at a low overall voltage of 1.55 V. This superior performance substantially satisfies the industrial requirement and paves the way to an efficient catalyst design for the UOR and green hydrogen production.


 Please wait while we load your content...
Please wait while we load your content...