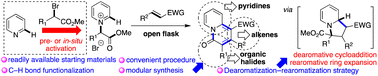
A dearomatization–rearomatization strategy for construction of 4H-quinolizin-4-ones via C–H bond functionalization of pyridines†
Abstract
Herein, based on a dearomatization–rearomatization strategy, a straightforward construction of poly-substituted and fused 4H-quinolizin-4-ones via direct ortho C–H bond functionalization of pyridines under metal-free conditions has been developed. This approach offers a convenient method for the modular synthesis of functionalized 4H-quinolizin-4-ones from simple pyridines, methyl 2-bromoacetates, and alkenes. Mechanistic studies indicated that the reaction included activation of pyridines to N-(2-methoxy-2-oxoethyl) pyridinium salts, dearomative [3 + 2] cycloaddition, and rearomative ring expansion processes. Notably, an aromatization-driven β cleavage of the relatively stable pyridine radical intermediate was proposed as a key process for the construction of the desired 4H-quinolizin-4-ones over the traditional indolizines.


 Please wait while we load your content...
Please wait while we load your content...