Improving the stability and selectivity for the oxygen-evolution reaction on semiconducting WO3 photoelectrodes with a solid-state FeOOH catalyst†
Abstract
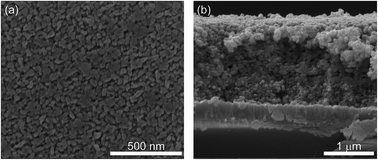
WO3 electrodes were synthesized via a sol–gel route followed by the photoelectrochemical deposition of a solid state FeOOH oxygen-evolution catalyst (OEC) to observe its effects on electrode stability and selectivity towards the oxygen evolution reaction (OER). WO3 photoanodes have been reported to degrade in aqueous solutions with pH > 3 due to the material's Arrhenius acidity and the potential formation of reactive peroxide intermediates on the WO3 surface during the course of photoelectrochemical water oxidation. The stability during photoelectrochemical OER of WO3 and WO3–FeOOH photoanodes was measured at 1.23 V vs. RHE at pH 4 and 7 in phosphate-buffered solutions. Additionally, the faradaic efficiencies of the electrodes for OER were measured at pH 4. WO3–FeOOH electrodes demonstrate a 95.9 ± 1.6% faradaic efficiency for OER in pH 4 potassium phosphate buffer at current densities of ∼0.75 mA cm−2 under 200 mW cm−2 AM1.5G illumination and an applied bias of 1.43 V vs. RHE. These experiments demonstrate that adding an FeOOH co-catalyst dramatically improves the stability of the electrodes, selectivity, and rate of OER versus that observed on WO3 films.
- This article is part of the themed collection: Water splitting and photocatalysis

 Please wait while we load your content...
Please wait while we load your content...