β-Trioxopyrrocorphins: pyrrocorphins of graded aromaticity†
Abstract
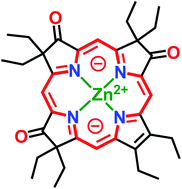
Octaethyltrioxopyrrocorphins unexpectedly show macrocycle-aromatic properties, even though they contain the macrocyclic π-system of the non-aromatic pyrrocorphins (hexahydroporphyrins). Two of the four possible triketone regioisomers were first reported in 1969 by one-pot oxidation of octaethylporphyrin but remained essentially unexplored since. We detail here the targeted preparation of the remaining two triketone isomers and the optical and NMR spectroscopic properties of all isomers. All four regioisomers possess unique electronic properties, including broadly varying degrees of diatropicity that were experimentally determined using 1H NMR spectroscopy and computationally verified. Structural patterns modulating the aromaticity were recognized. These differences highlight the regioisomerically differentiated influences of the three β-oxo-functionalities. We also present the solid state structure of the two most common isomers (in their free base form or as zinc complexes), allowing further conclusions to be made about the resonance structures present in these triketones. Remarkably, also, the halochromic properties of the triketones differ sharply from those of regular (hydro)porphyrins, providing further support for the proposed 16-membered, 18 π-electron aromatic ring-current. The work conceptually expands the understanding of tris-modified hydroporphyrinoid analogues and the factors that enable and control porphyrinoid aromaticity.
- This article is part of the themed collections: Emerging Frontiers in Aromaticity and 2021 Chemical Science HOT Article Collection


 Please wait while we load your content...
Please wait while we load your content...