Ruthenium-catalyzed formal sp3 C–H activation of allylsilanes/esters with olefins: efficient access to functionalized 1,3-dienes†
Abstract
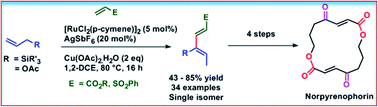
Ru-catalysed oxidative coupling of allylsilanes and allyl esters with activated olefins has been developed via isomerization followed by C(allyl)–H activation providing efficient access to stereodefined 1,3-dienes in excellent yields. Mild reaction conditions, less expensive catalysts, and excellent regio- and diastereoselectivity ensure universality of the reaction. In addition, the unique power of this reaction was illustrated by performing the Diels–Alder reaction, and enantioselective synthesis of highly functionalized cyclohexenone and piperidine and finally synthetic utility was further demonstrated by the efficient synthesis of norpyrenophorin, an antifungal agent.
- This article is part of the themed collection: 2021 Chemical Science HOT Article Collection


 Please wait while we load your content...
Please wait while we load your content...