Ru-catalyzed isomerization of ω-alkenylboronates towards stereoselective synthesis of vinylboronates with subsequent in situ functionalization†
Abstract
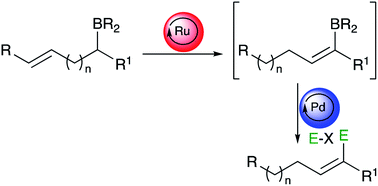
The stereoselective preparation of synthetically versatile vinylboronates from ω-alkenylboronates is achieved through a ruthenium-catalyzed isomerization reaction. A variety of di- and trisubstituted vinylboronates were conveniently produced and could be used as a new starting point for subsequent in situ remote functionalization through either a sequential Ru/Pd or Ru/Cu double catalytic system.
- This article is part of the themed collection: 2020 Chemical Science HOT Article Collection


 Please wait while we load your content...
Please wait while we load your content...