NaBH4 induces a high ratio of Ni3+/Ni2+ boosting OER activity of the NiFe LDH electrocatalyst†
Abstract
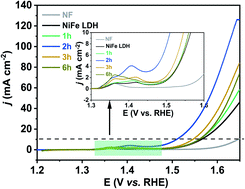
Electrochemical water splitting is a promising way to produce hydrogen gas, but the sluggish kinetics of the oxygen evolution reaction (OER) extremely restrict the overall conversion efficiency of water splitting. Transition metal based LDHs (TM LDHs) are one of the most effective non-noble metal OER catalysts and have attracted wide interest, especially the nickel–iron LDH (NiFe LDH). The high valence Ni3+ species with a large coordination number play a vital role in OER catalysis. Herein, we report on a surprising discovery that reaction between NiFe LDH and NaBH4 with multi-hydrides induces vacancy formation around Fe3+ and enrichment in Ni3+, crucially activating the OER performance. The ratio of Ni3+/Ni2+ is found to be closely tied to the OER performance, nicely accounting for the leading role of Ni3+ ions in octahedral sites in electrocatalysis. Significantly, the NaBH4 treated NiFe LDH directly on nickel foam (NF), denoted as NaBH4–NiFe LDH@NF exhibited an outstanding OER performance with an overpotential of only 310 mV at 100 mA cm−2, and a Tafel slope of 47 mV dec−1. For the series of TM LDHs we studied with different metal combinations, the high valence metal ion is found to be positively related to OER performance.
- This article is part of the themed collection: 2020 RSC Advances HOT Article Collection


 Please wait while we load your content...
Please wait while we load your content...