Regio- and stereoselective intermolecular carbolithiation reactions
Abstract
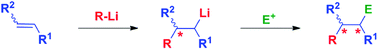
The inter-molecular carbolithiation of unfunctionalized and functionalized alkenes, followed by electrophile trapping, provides a versatile and useful tool for the construction of complex molecular systems, allowing the simultaneous creation of up to two novel carbon–carbon or carbon–heteroatom bonds. In this review a full overview on inter-molecular carbolithiation processes is reported, with a main focus on the chemo-, regio- and stereo-control of the reaction and on the strategies for asymmetric processes.
- This article is part of the themed collection: 2020 Reviews in RSC Advances


 Please wait while we load your content...
Please wait while we load your content...