One-pot synthesis of indoles and quinolinones from ortho-tosylaminophenyl-substituted para-quinone methides†
Abstract
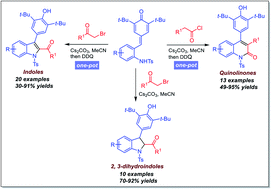
A facile one-pot synthesis has been developed through alkylation/acylation of ortho-tosylaminophenyl-substituted para-quinone methides followed by an intramolecular 1,6-conjugate addition and oxidation sequence. This cascade reaction occurs readily in good yield (up to 95%), providing a divergent synthetic approach to structurally diverse 2,3-disubstituted indoles and 3,4-diaryl-substituted quinolinones.
- This article is part of the themed collection: 2020 RSC Advances HOT Article Collection


 Please wait while we load your content...
Please wait while we load your content...