Stereoselective synthesis of the head group of archaeal phospholipid PGP-Me to investigate bacteriorhodopsin–lipid interactions†
Abstract
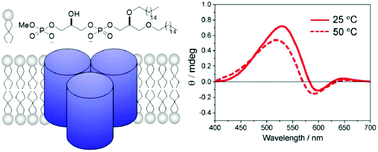
Phosphatidylglycerophosphate methyl ester (PGP-Me), a major constituent of the archaeal purple membrane, is essential for the proper proton-pump activity of bacteriorhodopsin (bR). We carried out the first synthesis of the bisphosphate head group of PGP-Me using H-phosphonate chemistry that led to the production of a simplified PGP-Me analogue with straight alkyl chains. To investigate the role of this head group in the structural and functional integrity of bR, the analogue was used to reconstitute bR into liposomes, in which bR retained the original trimeric structure and light-induced photocycle activity. Enhanced ordering of an alkyl chain of the 2H-labelled analogue was observed in 2H NMR spectra upon interaction with bR. These results together suggest that the bisphosphate moiety plays a role in the proper functioning of bR through the lipid–protein interaction.

 Please wait while we load your content...
Please wait while we load your content...