Fluorenone core donor–acceptor–donor π-conjugated molecules end-capped with dendritic oligo(thiophene)s: synthesis, liquid crystalline behaviour, and photovoltaic applications†
Abstract
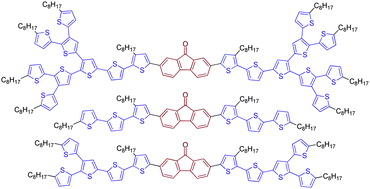
We have synthesized a new series of donor–acceptor–donor (D–A–D) π-conjugated molecules, consisting of fluorenone core end-capped with dendritic oligo(thiophene)s of increasing generation (abbreviated as FG0, FG1, and FG2). In view of the application of these new organic semiconductors in photovoltaic devices, we have explored their spectroscopic, redox, and structural properties. The thermal behaviour of the new organic semiconductors was investigated by

 Please wait while we load your content...
Please wait while we load your content...