A multidisciplinary investigation of the technical and environmental performances of TAML/peroxide elimination of Bisphenol A compounds from water†‡
Abstract
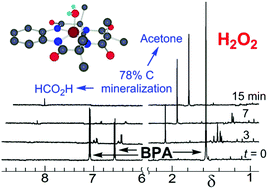
Designing technologies that mitigate the low-dose adverse effects of exposures to large-volume, everyday-everywhere chemicals such as bisphenol A (BPA, 1a) requires an understanding of the scope of the exposures and the nature of the adverse effects. Therefore, we review the literature of, (i) the occurrences of 1a in humans, waters and products and the effectiveness of widely deployed mitigation methods in 1a stewardship and, (ii) the adverse effects of 1a exposures on human cells and fish. Within this broad context, we present and evaluate experimental results on TAML/H2O2 purification of 1a contaminated waters. TAML/H2O2 catalysis readily oxidizes BPA (1a) and the ring-tetramethyl (1b), tetrachloro (1c), and tetrabromo (1d)-substituted derivatives. At pH 8.5, TAML/H2O2 induces controllable, oxidative oligomerisation of 1a (2-, 3-, 4-, and 5-unit species were identified) with precipitation, establishing a green synthetic pathway to these substances for biological safety characterisation and an easy method for near quantitative removal of 1a from water. TAML/H2O2 (24 nM/4 mM) treatment of 1a (10 000 μg L−1) in pH 8.5 (0.01 M, carbonate) lab water effects a >99% reduction (to <100 μg L−11a) within 30 min. Yeast oestrogen screens (YES) of the pH 8.5, TAML/H2O2 treated, catalase quenched, and filtered oxidation solutions show elimination of 1a oestrogenicity. Zebrafish developmental assays of TAML/H2O2 treated, unfiltered, agitated pH 7, 1a solutions showed no significant incidences of abnormality among any of 22 endpoints—treated samples showed an insignificant increase in mortality. At pH 11, the TAML/H2O2 oxidations of 1a–d are fast with second order rate constants for the substrate oxidation process (kII values) of (0.57–8) × 104 M−1 s−1. The 1a oxidation gives CO and CO2 (∼78%), acetone (∼25%) and formate (∼1%). In striking contrast with pH 8.5 treatment, no oligomers were detected. TAML/H2O2 (150 nM/7.5 mM) treatment of 1a (34 244 μg L−1) in pH 11 (0.01 M, phosphate) lab water effected a >99.9% reduction (to <23 μg L−11a) within 15 min. The pH dependent behaviour of 1a was examined as a possible origin of the differing outcomes. The 1st and 2nd pKa values of 1a were estimated by fitting the pH dependence of the UV-vis spectra (pKa1 = 9.4 ± 0.3; pKa2 = 10.37 ± 0.07). At pH 8.5, coupling of the radical produced on initial oxidation evidently outcompetes further oxidation. A linear free energy relationship between the logarithm of the pH 11, kII values and the redox potentials of 1a–d as determined by differential pulse voltammetry in CH3CN is consistent with rate-limiting, electron transfer from the dianionic form of 1a at pH 11, followed by a multistep, deep degradation without observation of 4-(prop-1-en-2-yl)phenol 12, a common 1a oxidation product—an improved synthesis of 12 is described. Microtox® analyses of pH 12, TAML/H2O2 treated 1a solutions showed significantly reduced toxicity. The facility and high efficiency by which TAML/H2O2 catalysis eliminates 1a from water, by either mechanism, suggests a new and simple procedure for 1a stewardship.


 Please wait while we load your content...
Please wait while we load your content...