Photoinduced hole transfer from tris(bipyridine)ruthenium dye to a high-valent iron-based water oxidation catalyst†
Abstract
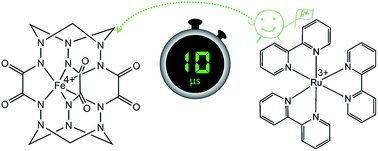
An efficient water oxidation system is a prerequisite for developing solar energy conversion devices. Using advanced time-resolved spectroscopy, we study the initial catalytic relevant electron transfer events in the light-driven water oxidation system utilizing [Ru(bpy)3]2+ (bpy = 2,2′-bipyridine) as a light harvester, persulfate as a sacrificial electron acceptor, and a high-valent iron clathrochelate complex as a catalyst. Upon irradiation by visible light, the excited state of the ruthenium dye is quenched by persulfate to afford a [Ru(bpy)3]3+/SO4˙− pair, showing a cage escape yield up to 75%. This is followed by the subsequent fast hole transfer from [Ru(bpy)3]3+ to the FeIV catalyst to give the long-lived FeV intermediate in aqueous solution. In the presence of excess photosensitizer, this process exhibits pseudo-first order kinetics with respect to the catalyst with a rate constant of 3.2(1) × 1010 s−1. Consequently, efficient hole scavenging activity of the high-valent iron complex is proposed to explain its high catalytic performance for water oxidation.
- This article is part of the themed collection: Artificial photosynthesis


 Please wait while we load your content...
Please wait while we load your content...