Is zeroth order crystal structure prediction (CSP_0) coming to maturity? What should we aim for in an ideal crystal structure prediction code?
Abstract
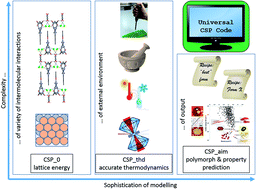
Crystal structure prediction based on searching for the global minimum in the lattice energy (CSP_0) is growing in use for guiding the discovery of new materials, for example, new functional materials, new phases of interest to planetary scientists and new polymorphs relevant to pharmaceutical development. This Faraday Discussion can assess the progress of CSP_0 over the range of types of materials to which CSP is currently and could be applied, which depends on our ability to model the variety of interatomic forces in crystals. The basic hypothesis, that the outcome of crystallisation is determined by thermodynamics, needs examining by considering methods of modelling relative thermodynamic stability not only as a function of pressure and temperature, but also of size, solvent and the presence of heterogeneous templates or impurities (CSP_thd). Given that many important materials persist, and indeed may be formed, when they are not the most thermodynamically stable structure, we need to define what would be required of an ideal CSP code (CSP_aim).
- This article is part of the themed collection: Methods and applications of crystal structure prediction


 Please wait while we load your content...
Please wait while we load your content...