Local, solvation pressures and conformational changes in ethylenediamine aqueous solutions probed using Raman spectroscopy†
Abstract
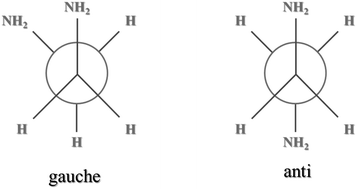
Raman spectra of 1,2-ethylenediamine (EDA) in aqueous solutions are used to demonstrate that EDA molecules experience an anti–gauche conformational change resulting from the interactions with water. The observed Raman shift reveals a compressive (hydrophobic) effect of water on both methylene and amino groups of EDA. Raman spectra of EDA at high pressures are used as reference to quantify the intermolecular EDA–H2O interactions in terms of local pressures. These results are compared with macroscopic solvation pressures calculated from the cohesive energy parameter. We compare and discuss all our observations with available computational and experimental studies.

 Please wait while we load your content...
Please wait while we load your content...