Pd-Catalyzed oxidative isomerization of propargylic acetates: highly efficient access to α-acetoxyenones via alkenyl Csp2–O bond-forming reductive elimination from PdIV
Abstract
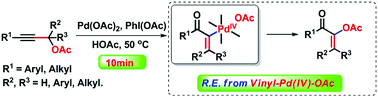
A Pd(II)/(IV)-catalyzed oxidative isomerization of propargylic acetates developed for the synthesis of polysubstituted alkenyl acetates is described. The reductive elimination of alkenyl Csp2–OAc bonds from PdIV intermediates is achieved. Mechanistic studies indicate that the reaction mechanism consists of trans acetoxypalladation of a triple bond, isomerization, oxidative addition with PhI(OAc)2 and alkenyl C–OAc bond reductive elimination.

 Please wait while we load your content...
Please wait while we load your content...