Combination of inverse electron-demand Diels–Alder reaction with highly efficient oxime ligation expands the toolbox of site-selective peptide conjugations†
Abstract
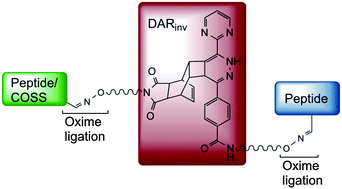
A modular approach combining inverse electron-demand Diels–Alder coupling (DARinv) and oxime ligation expands the toolbox of bioorthogonal peptide chemistry. Applicability of versatile site-specific bifunctional building blocks is demonstrated by generation of defined conjugates comprising linear, cystine-bridged and multi-disulfide functional peptides as well as their conjugation with hybrid silsesquioxane nanoparticles.

 Please wait while we load your content...
Please wait while we load your content...