Self-validated detection of hydroquinone in medicated cosmetic products using a preanodized screen-printed ring disk carbon electrode
Abstract
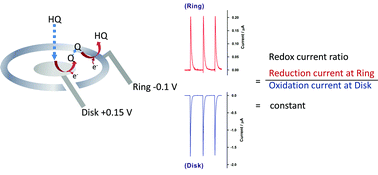
An anodically pretreated screen-printed ring disk carbon electrode (SPRDCE*) coupled with a flow injection analysis system was developed as a simple, rapid, sensitive, and self-validated hydroquinone (HQ) sensor. HQ was electrocatalytically oxidized at the preanodized disk electrode and the oxidized product para-benzoquinone was then electrocatalytically reduced to HQ at the preanodized ring electrode. A well-defined and quasireversible redox peak couple was observed. The amount of HQ could be determined by using the catalytic current from the SPRDCE*. Besides, it is also worth noting that the performance of this system could be validated directly by using the redox current ratio (the reduction current at the ring electrode divided by the oxidation current at the disk electrode). Under optimized conditions, a linear range for HQ at the disk electrode was in the range of 0.25–160 ppm with 0.999 and 0.024 ppm for the correlation coefficient and the detection limit (S/N = 3), respectively. Using this method, HQ in cosmetic products was successfully quantified without any pre-treatment with recoveries between 97.76% and 103.74%.

 Please wait while we load your content...
Please wait while we load your content...