Theory for heterogeneous electron transfer in self-assembled monolayers on metal electrodes
Abstract
We develop a semi-microscopic theory for the heterogeneous electron transfer (HET) rate constant (k0) based on the premise of the energy level alignment approach of matching the fluctuating quasi-Fermi level of the metal with the fluctuating frontier molecular orbital (FMO) of electroactive species at the self-assembled monolayer (SAM) covered metal electrodes. k0 is modeled through the free energy of activation (ΔG≠), which is a product of the interfacial electron affinity ratio of the electrode interface 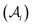 , and the energy gap (Δ) between the electrode and electroactive molecule, moderated through solvent reorganization energy (λs).
, and the energy gap (Δ) between the electrode and electroactive molecule, moderated through solvent reorganization energy (λs).  can be modulated through the electronic properties and defects of the metal, charge reorganization (
can be modulated through the electronic properties and defects of the metal, charge reorganization (![[small delta, Greek, tilde]](https://www.rsc.org/images/entities/i_char_e125.gif) ) by the molecular dipole, dipole moment, and composition of the adsorbing molecule. The charge reorganization over the metal surface by the alkanethiols is 9–13% of the electronic charge. A novel microscopic approach is developed for evaluating solvent reorganization energy as the interaction between the redox ion and dipoles. k0 is a function of the electronic properties of the metal jellium (screening length, dielectric constant), characteristics of dipolar SAM (packing density, spacer length, and dipole moment), and the FMO energies of the electroactive molecule. The theory accounts for the influence of the spacer length of the alkanethiol and temperature on the HET kinetics. Finally, the theory shows agreement with the reported experimental data of the HET kinetics for the ferrocenyl-n-alkanethiols monolayer, Fc(CH2)nSH (n = 5, 6, 8, 9, and 11) and alkanethiols in the presence of [Ru(NH3)6]+2/+3 on Au(111) and Hg, respectively.
) by the molecular dipole, dipole moment, and composition of the adsorbing molecule. The charge reorganization over the metal surface by the alkanethiols is 9–13% of the electronic charge. A novel microscopic approach is developed for evaluating solvent reorganization energy as the interaction between the redox ion and dipoles. k0 is a function of the electronic properties of the metal jellium (screening length, dielectric constant), characteristics of dipolar SAM (packing density, spacer length, and dipole moment), and the FMO energies of the electroactive molecule. The theory accounts for the influence of the spacer length of the alkanethiol and temperature on the HET kinetics. Finally, the theory shows agreement with the reported experimental data of the HET kinetics for the ferrocenyl-n-alkanethiols monolayer, Fc(CH2)nSH (n = 5, 6, 8, 9, and 11) and alkanethiols in the presence of [Ru(NH3)6]+2/+3 on Au(111) and Hg, respectively.

- This article is part of the themed collection: Structure and dynamics of chemical systems: Honouring N. Sathyamurthy’s 75th birthday


 Please wait while we load your content...
Please wait while we load your content...