Phase equilibrium and dynamics of 5CB mixed with dimethyl terephthalate: coupling of orientation and composition fluctuations in isotropic phase†
Abstract
4-Cyano-4′-pentylbiphenyl (5CB) is a representative liquid crystalline compound undergoing isotropic-to-nematic phase transition at a critical temperature,  . This phase behavior significantly changes on addition of a small amount of a second compound, and the dynamics of 5CB molecules changes accordingly. In this study, these changes were examined for mixtures of 5CB with dimethyl terephthalate (DMT), the latter being in the crystalline state at T < 415 K if not mixed with 5CB, and the results were utilized to discuss dynamic properties of the mixture, the kinematic viscosity and dielectric relaxation time. Experiments showed that the mixtures with low DMT content wDMT (<3.2 wt%) exhibited the isotropic-to-nematic transition at TIN and this TIN gradually decreased to 305 K on an increase of wDMT up to 3.2 wt%. On further cooling to TNC (<TIN), pure DMT crystallite precipitated from the nematic phase. On the other hand, for the mixture with larger wDMT (>3.2 wt%), the DMT crystallite precipitated from the isotropic phase on cooling to TIC, and this TIC strongly increased on an increase of wDMT (which reflected a finite solubility of DMT in the isotropic phase). On further cooling to
. This phase behavior significantly changes on addition of a small amount of a second compound, and the dynamics of 5CB molecules changes accordingly. In this study, these changes were examined for mixtures of 5CB with dimethyl terephthalate (DMT), the latter being in the crystalline state at T < 415 K if not mixed with 5CB, and the results were utilized to discuss dynamic properties of the mixture, the kinematic viscosity and dielectric relaxation time. Experiments showed that the mixtures with low DMT content wDMT (<3.2 wt%) exhibited the isotropic-to-nematic transition at TIN and this TIN gradually decreased to 305 K on an increase of wDMT up to 3.2 wt%. On further cooling to TNC (<TIN), pure DMT crystallite precipitated from the nematic phase. On the other hand, for the mixture with larger wDMT (>3.2 wt%), the DMT crystallite precipitated from the isotropic phase on cooling to TIC, and this TIC strongly increased on an increase of wDMT (which reflected a finite solubility of DMT in the isotropic phase). On further cooling to 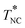 (= 305 K), the liquid phase being in equilibrium with the DMT crystallite changed from the isotropic phase to the nematic phase. This
(= 305 K), the liquid phase being in equilibrium with the DMT crystallite changed from the isotropic phase to the nematic phase. This 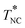 was independent of wDMT and coincided with the other transition temperatures TIC, TIN, and TNC merging at wDMT = 3.2 wt%. All these features, cast in a phase diagram, were well described by a simple free energy model incorporating the Flory–Huggins type mixing free energy, the Landau–de Gennes type nematic free energy, and the crystal dissolution energy. Corresponding to this phase behavior, the kinematic viscosity ν and the dielectric relaxation time τε of the 5CB/DMT mixtures exhibited Eyring–Andrade type temperature dependence in the high-T asymptote (in the isotropic one phase state) but positively deviated from respective high-T asymptotes on cooling to TIN (for wDMT = 1.5 wt%) and/or to
was independent of wDMT and coincided with the other transition temperatures TIC, TIN, and TNC merging at wDMT = 3.2 wt%. All these features, cast in a phase diagram, were well described by a simple free energy model incorporating the Flory–Huggins type mixing free energy, the Landau–de Gennes type nematic free energy, and the crystal dissolution energy. Corresponding to this phase behavior, the kinematic viscosity ν and the dielectric relaxation time τε of the 5CB/DMT mixtures exhibited Eyring–Andrade type temperature dependence in the high-T asymptote (in the isotropic one phase state) but positively deviated from respective high-T asymptotes on cooling to TIN (for wDMT = 1.5 wt%) and/or to 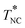 (for wDMT = 4.5 wt%). Pure 5CB exhibited similar positive deviation but in a much narrower range of T (just in a close vicinity of
(for wDMT = 4.5 wt%). Pure 5CB exhibited similar positive deviation but in a much narrower range of T (just in a close vicinity of 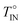 ). This difference between the 5CB/DMT mixture and pure 5CB was attributed to the composition fluctuation (missing in pure 5CB) strongly coupled with the orientation fluctuation in the isotropic phase of the mixture, and this coupling was assigned as the origin of the gradual/broad deviation observed for both ν and τε. On the basis of this assignment, the fluctuation of the free energy was analyzed with the aid of the model explained above. This analysis specified a relationship between the deviations of ν and τε, and the ν and τε data well obeyed this relationship. These results demonstrate the importance of the coupling of the composition and orientation fluctuations in the dynamic properties of 5CB/DMT mixtures.
). This difference between the 5CB/DMT mixture and pure 5CB was attributed to the composition fluctuation (missing in pure 5CB) strongly coupled with the orientation fluctuation in the isotropic phase of the mixture, and this coupling was assigned as the origin of the gradual/broad deviation observed for both ν and τε. On the basis of this assignment, the fluctuation of the free energy was analyzed with the aid of the model explained above. This analysis specified a relationship between the deviations of ν and τε, and the ν and τε data well obeyed this relationship. These results demonstrate the importance of the coupling of the composition and orientation fluctuations in the dynamic properties of 5CB/DMT mixtures.



 Please wait while we load your content...
Please wait while we load your content...