Enhanced discharge capacity of thermorechargeable batteries composed of Cd-PBA and a potential-matched PBA†
Abstract
Thermorechargeable batteries (TBs), which can be charged by a temperature change ΔT via the difference in the electrochemical Seebeck coefficient α between the cathode and anode, are promising energy harvesters. In order to put TBs into practical use, it is necessary to increase the discharge capacity (QTB) per unit weight of the total active materials, which is governed by the temperature (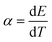 ; T is temperature) and capacity (
; T is temperature) and capacity (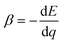 ; q is capacity) coefficients of the potential E of cathode and anode materials. Among the active materials, the cadmium Prussian blue analogue (Cd-PBA) with a large |α| and small β is an ideal anode material for TBs. Here, we developed a potential-matched cathode PBA by partially substituting the Co sites of Co-PBA with other transition metals. We found that the potential of Mn-substituted Co-PBA matches well with that of Cd-PBA. We demonstrated that QTB (=4.1–5.1 mA h g−1) of CoMn-PBA/Cd-PBA TB is much larger than that (=0.7–1.1 mA h g−1) of non-substituted Co-PBA/Cd-PBA TBs.
; q is capacity) coefficients of the potential E of cathode and anode materials. Among the active materials, the cadmium Prussian blue analogue (Cd-PBA) with a large |α| and small β is an ideal anode material for TBs. Here, we developed a potential-matched cathode PBA by partially substituting the Co sites of Co-PBA with other transition metals. We found that the potential of Mn-substituted Co-PBA matches well with that of Cd-PBA. We demonstrated that QTB (=4.1–5.1 mA h g−1) of CoMn-PBA/Cd-PBA TB is much larger than that (=0.7–1.1 mA h g−1) of non-substituted Co-PBA/Cd-PBA TBs.



 Please wait while we load your content...
Please wait while we load your content...