Chemoselective sulfonyl fluoride exchange (SuFEx)-induced macrocyclization of tyrosine-containing peptides in aqueous media
Abstract
Cyclic peptides generally exhibit enhanced metabolic stability, cell permeability, and binding affinity to biological targets compared to linear peptide sequences, making them attractive scaffolds for therapeutic development. Due to the chemical heterogeneity of peptides, the development of new, chemoselective methods for peptide macrocyclization remains a significant challenge. Here, we report a tyrosine-selective strategy for the synthesis of 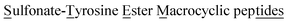 (STEMtides) in aqueous buffer under mild conditions. This method leverages sulfur fluoride exchange (SuFEx) chemistry to engage the phenolic side chain of tyrosine residues with sulfonyl fluorides, achieving efficient and chemoselective cyclization without the need for additional reagents. The approach is highly tolerant of native side chain functionality and enables access to cyclic peptides ranging from 2 to 13 residues in length. To demonstrate the scope and translational potential of the method, STEMtide analogs of several clinically relevant peptides, including leuprorelin, β-MSH, liraglutide, and cilengitide, a cyclic RGD peptidomimetic, were successfully synthesized in high yield using the SuFEx-mediated strategy. RGD STEMtide analogs exhibited low toxicity to MCF-7 cells, as well as potent inhibition of cell adhesion comparable to cilengitide itself, highlighting the therapeutic potential of this new class of peptide macrocycles.
(STEMtides) in aqueous buffer under mild conditions. This method leverages sulfur fluoride exchange (SuFEx) chemistry to engage the phenolic side chain of tyrosine residues with sulfonyl fluorides, achieving efficient and chemoselective cyclization without the need for additional reagents. The approach is highly tolerant of native side chain functionality and enables access to cyclic peptides ranging from 2 to 13 residues in length. To demonstrate the scope and translational potential of the method, STEMtide analogs of several clinically relevant peptides, including leuprorelin, β-MSH, liraglutide, and cilengitide, a cyclic RGD peptidomimetic, were successfully synthesized in high yield using the SuFEx-mediated strategy. RGD STEMtide analogs exhibited low toxicity to MCF-7 cells, as well as potent inhibition of cell adhesion comparable to cilengitide itself, highlighting the therapeutic potential of this new class of peptide macrocycles.



 Please wait while we load your content...
Please wait while we load your content...