Fluorinated and methylated ortho-benzodipyrrole-based acceptors suppressing charge recombination and minimizing energy loss in organic photovoltaics†
Abstract
The elimination of the A′ unit from 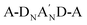 -type Y6-derivatives has led to the development of a new class of ortho-benzodipyrrole (o-BDP)-based A-DNBND-A-type NFAs. In this work, two new A-DNBND-A-type NFAs, denoted as CFB and CMB, are designed and synthesized, where electron-withdrawing fluorine atoms and electron-donating methyl groups are substituted on the benzene ring of the o-BDP moiety, respectively. CFB exhibits a blue-shifted absorption spectrum, stronger intermolecular interactions, shorter π–π stacking distances, and more ordered 3D intermolecular packing in the neat and blend films, enabling it to effectively suppress charge recombination in the PM6:CFB device showing a higher PCE of 16.55% with an FF of 77.45%. CMB displays a higher HOMO/LUMO energy level, a smaller optical bandgap, and a less ordered 3D packing, which contributes to its superior ability to suppress energy loss in the PM6:CMB device with a high Voc of 0.90 V and a PCE of 16.46%. To leverage the advantages of CFB and CMB, ternary PM6:Y6-16:CFB and PM6:Y6-16:CMB devices are fabricated. The PM6:Y6-16:CFB device exhibits the highest PCE of 17.83% with an increased Voc of 0.86 V and a Jsc of 27.32 mA cm−2, while the PM6:Y6-16:CMB device displayed an elevated Voc of 0.87 V and an improved FF of 74.71%, leading to a PCE of 17.44%. The high PCE was achieved using the non-halogenated greener solvent o-xylene, highlighting their potential for facilitating more eco-friendly processing procedures. C-shaped disubstituted o-BDP-based A–D–A type acceptors open up new avenues for tailoring electronic properties and molecular self-assembly, achieving higher OPV performance with enhanced charge recombination suppression and reduced energy loss.
-type Y6-derivatives has led to the development of a new class of ortho-benzodipyrrole (o-BDP)-based A-DNBND-A-type NFAs. In this work, two new A-DNBND-A-type NFAs, denoted as CFB and CMB, are designed and synthesized, where electron-withdrawing fluorine atoms and electron-donating methyl groups are substituted on the benzene ring of the o-BDP moiety, respectively. CFB exhibits a blue-shifted absorption spectrum, stronger intermolecular interactions, shorter π–π stacking distances, and more ordered 3D intermolecular packing in the neat and blend films, enabling it to effectively suppress charge recombination in the PM6:CFB device showing a higher PCE of 16.55% with an FF of 77.45%. CMB displays a higher HOMO/LUMO energy level, a smaller optical bandgap, and a less ordered 3D packing, which contributes to its superior ability to suppress energy loss in the PM6:CMB device with a high Voc of 0.90 V and a PCE of 16.46%. To leverage the advantages of CFB and CMB, ternary PM6:Y6-16:CFB and PM6:Y6-16:CMB devices are fabricated. The PM6:Y6-16:CFB device exhibits the highest PCE of 17.83% with an increased Voc of 0.86 V and a Jsc of 27.32 mA cm−2, while the PM6:Y6-16:CMB device displayed an elevated Voc of 0.87 V and an improved FF of 74.71%, leading to a PCE of 17.44%. The high PCE was achieved using the non-halogenated greener solvent o-xylene, highlighting their potential for facilitating more eco-friendly processing procedures. C-shaped disubstituted o-BDP-based A–D–A type acceptors open up new avenues for tailoring electronic properties and molecular self-assembly, achieving higher OPV performance with enhanced charge recombination suppression and reduced energy loss.

- This article is part of the themed collection: Most popular 2025 energy conversion articles


 Please wait while we load your content...
Please wait while we load your content...