Chirality inversion in cholesteric phases of bent-shaped liquid crystal dimers with chiral dopants
Abstract
Incorporating molecular chirality into liquid crystals (LCs) yields nanoscale one-handed helical structures. The helical pitch of a cholesteric (Ch) phase (i.e., a chiral nematic phase) is typically slightly temperature-dependent, and the sense of helical rotation (i.e., chirality) rarely inverts. This study reports helical pitch divergence and chirality inversion in the Ch phase of bent-shaped LC dimers added with a chiral dopant (CD). We prepared four chiral LC mixtures containing equal proportions of two ester-linked 4-(trans-4-pentylcyclohexyl)phenyl-based bent-shaped LC dimers with odd-numbered spacers, each doped with 0.25, 0.50, 1.0, or 2.0 wt% of an isosorbide-based CD, ISO-(6OBA)2. All mixtures exhibited enantiotropic chiral twist-bend nematic 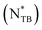 and Ch phases near room temperature. Polarized optical microscopy revealed that in the Ch phases of the dimer mixtures with 0.25, 0.50, and 1.0 wt% CD, the helix diverged and reformed with opposite chirality just above the
and Ch phases near room temperature. Polarized optical microscopy revealed that in the Ch phases of the dimer mixtures with 0.25, 0.50, and 1.0 wt% CD, the helix diverged and reformed with opposite chirality just above the 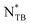 phase transition temperature. The high- and low-temperature Ch phases were identified as right- and left-handed, respectively, using circular dichroism spectroscopy and the contact preparation method. The dimer mixture with 2.0 wt% CD exhibited only helix divergence of the right-handed Ch phase. These phenomena were also observed in each nonblended dimer, but not in other bent-shaped cyanobiphenyl-based twist-bend nematogenic dimers with different linkages. Our findings offer new insights into chirality inversion in LCs and have promising potential for temperature-tunable chiral-switching systems based on bent-shaped LC dimers.
phase transition temperature. The high- and low-temperature Ch phases were identified as right- and left-handed, respectively, using circular dichroism spectroscopy and the contact preparation method. The dimer mixture with 2.0 wt% CD exhibited only helix divergence of the right-handed Ch phase. These phenomena were also observed in each nonblended dimer, but not in other bent-shaped cyanobiphenyl-based twist-bend nematogenic dimers with different linkages. Our findings offer new insights into chirality inversion in LCs and have promising potential for temperature-tunable chiral-switching systems based on bent-shaped LC dimers.



 Please wait while we load your content...
Please wait while we load your content...