Electrochemical, surface, DFT, and ADMET insights into (E)-2-(2-hydroxybenzylidene)hydrazine-1-carboxamide as a corrosion inhibitor
Abstract
In this work, (E)-2-((2-hydroxybenzylidene))hydrazine-1-carboxamide (HBHC) was investigated as a new organic and eco-friendly corrosion inhibitor for mild steel in acidic medium through electrochemical measurements including potentiodynamic polarization (PP), electrochemical impedance spectroscopy (EIS), long-term immersion tests, surface characterization, theoretical calculations, and ADMET studies. HBHC demonstrated excellent inhibition performance, achieving 94.50% efficiency by PP and 93.33% by EIS at 200 ppm, and retained remarkable stability over 30 days of immersion with 97.64% efficiency. Adsorption behavior was consistent with the Langmuir isotherm, with negative 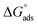 values indicating a mixed physisorption–chemisorption mechanism. SEM micrographs, EDX analysis, and elemental mapping analysis confirmed the formation of a uniform protective film enriched with heteroatoms on the steel surface. DFT calculations, including analysis of HOMO–LUMO frontier orbitals, revealed a low HOMO–LUMO energy gap (ΔE), supporting the high reactivity of HBHC and its strong donor–acceptor interactions with the Fe(110) surface, while MD simulations further confirmed its adsorption stability. Furthermore, ADMET predictions indicated low toxicity and good bioavailability, supporting the environmentally benign character of HBHC.
values indicating a mixed physisorption–chemisorption mechanism. SEM micrographs, EDX analysis, and elemental mapping analysis confirmed the formation of a uniform protective film enriched with heteroatoms on the steel surface. DFT calculations, including analysis of HOMO–LUMO frontier orbitals, revealed a low HOMO–LUMO energy gap (ΔE), supporting the high reactivity of HBHC and its strong donor–acceptor interactions with the Fe(110) surface, while MD simulations further confirmed its adsorption stability. Furthermore, ADMET predictions indicated low toxicity and good bioavailability, supporting the environmentally benign character of HBHC.



 Please wait while we load your content...
Please wait while we load your content...