Reusing the expired Ceftazidime drug as an inhibiting agent for zinc metal corrosion in HCl medium†
Abstract
Drug disposal costs and drug-related environmental pollution could be reduced by reusing expired drugs. A well-known cephalosporin antibiotic that was listed as an essential medication by the World Health Organization is Ceftazidime. In this study, the corrosion mitigation properties of the expired Ceftazidime (CDZ) drug for zinc corrosion in 1 mol per L HCl solution were examined by weight loss (WL), frequency modulation (EFM), potentiodynamic polarization (PDP), and impedance spectroscopy (EIS) techniques. Theoretical calculations were carried out by Density Functional Tight Binding (DFTB) and Monte Carlo (MC) simulations. Furthermore, scanning electron microscopy (SEM) and energy dispersion X-rays (EDX) were used for examining the appearance and structure of the corroded zinc surface, respectively. For a CDZ concentration of 300 ppm, EFM techniques have shown an inhibitive efficiency (IE) of 70.3% at 298 K. The IE increased as the drug concentration increased from 50 to 300 ppm, whereas it reduced as the temperature increased. The inhibition effect ceased to improve at concentrations greater than 300 ppm. A mixed form of adsorption (physisorption and chemisorption) was suggested for inhibition. The adsorption process followed the Temkin model. The spontaneous character and exothermicity of the adsorption process were demonstrated by the 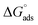 and Kads magnitudes. The PDP study demonstrated that CDZ was a mixed-type inhibitor as it retards both the cathodic and anodic reactions. According to EFM results, increasing the concentration of CDZ from 50 to 300 ppm reduces the corrosion current density from 163.5 to 78.7 μA cm−2. SEM and EDX examinations revealed the adsorption of CDZ drug at the zinc surface. The DFTB and MC simulations revealed that the CDZ drug bonds to the zinc surface via the heteroatoms' lone pair of electrons and the pyridinium ring's π-electrons. The adsorption energy of the drug on the Zn surface was found to be −77.41 kJ mol−1.
and Kads magnitudes. The PDP study demonstrated that CDZ was a mixed-type inhibitor as it retards both the cathodic and anodic reactions. According to EFM results, increasing the concentration of CDZ from 50 to 300 ppm reduces the corrosion current density from 163.5 to 78.7 μA cm−2. SEM and EDX examinations revealed the adsorption of CDZ drug at the zinc surface. The DFTB and MC simulations revealed that the CDZ drug bonds to the zinc surface via the heteroatoms' lone pair of electrons and the pyridinium ring's π-electrons. The adsorption energy of the drug on the Zn surface was found to be −77.41 kJ mol−1.



 Please wait while we load your content...
Please wait while we load your content...