A redox reaction triggered by hydrostatic pressure in dicationic cyclophanes†
Abstract
Various reactions and systems that respond to hydrostatic pressure, i.e., one type of mechanical isotropic stimulus, have been developed over the past decades. Here, we show that a one-electron (1e) reduction of dicationic cyclophane can be realised by applying hydrostatic pressure in a water-containing solvent. The large negative value of the volume change 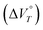 observed for this reduction, which is key to inducing the reduction reaction, is due to the desolvation of the H2O molecules and the change in the proximity between the cyclophane π units accompanied by a decrease in electrostatic repulsion. In fact, related monocations did not undergo a 1e reduction under hydrostatic pressure, even in water-containing solvents, indicating that the reduction behaviour is enabled by the cyclophane structure. Furthermore, in the case of weakly polar anions such as BF4− and PF6−, a change in the solvation/desolvation of the H2O molecules of dicationic cyclophanes can occur upon hydrostatic pressurisation, leading to a 1e reduction, showing that the reduction behaviour can be tuned by selecting the appropriate counter anion. Therefore, this study provides a valuable strategy and guidelines for the rational design of molecules with redox behaviour that can be modulated using hydrostatic pressure.
observed for this reduction, which is key to inducing the reduction reaction, is due to the desolvation of the H2O molecules and the change in the proximity between the cyclophane π units accompanied by a decrease in electrostatic repulsion. In fact, related monocations did not undergo a 1e reduction under hydrostatic pressure, even in water-containing solvents, indicating that the reduction behaviour is enabled by the cyclophane structure. Furthermore, in the case of weakly polar anions such as BF4− and PF6−, a change in the solvation/desolvation of the H2O molecules of dicationic cyclophanes can occur upon hydrostatic pressurisation, leading to a 1e reduction, showing that the reduction behaviour can be tuned by selecting the appropriate counter anion. Therefore, this study provides a valuable strategy and guidelines for the rational design of molecules with redox behaviour that can be modulated using hydrostatic pressure.



 Please wait while we load your content...
Please wait while we load your content...