Can we predict specific numbers of catalytically important molecules of water in H/D exchange in aromatic systems? A combined NMR and DFT study†
Abstract
Base-catalyzed H/D exchange reactions through keto–enol tautomeric equilibrium are a textbook example in mechanistic organic chemistry. The pH effect of H2O catalysis, however, is largely unknown. We report, herein, variable temperature and pD 1H NMR studies of the experimental activation enthalpy  , entropy
, entropy 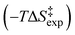 , and Gibbs free energy
, and Gibbs free energy 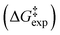 of H/D exchange reactions of the H-6 and H-8 protons belonging to ring A of the natural product taxifolin. The experimental
of H/D exchange reactions of the H-6 and H-8 protons belonging to ring A of the natural product taxifolin. The experimental 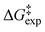 values range from ∼25 to 23 kcal mol−1 for pD values of 6.1 to 9.6 and a buffer concentration in the range of 25 to 1000 mM. Differences in
values range from ∼25 to 23 kcal mol−1 for pD values of 6.1 to 9.6 and a buffer concentration in the range of 25 to 1000 mM. Differences in 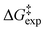 values of neutral and anionic taxifolin and phloroglucinol were found to be very small (≤1.5 kcal mol−1). The experimental data of taxifolin and phloroglucinol were compared with DFT calculations with two up to four H2O molecules explicitly present, which demonstrate a unique catalytic role of H2O of over 35 kcal mol−1. Excellent agreement between
values of neutral and anionic taxifolin and phloroglucinol were found to be very small (≤1.5 kcal mol−1). The experimental data of taxifolin and phloroglucinol were compared with DFT calculations with two up to four H2O molecules explicitly present, which demonstrate a unique catalytic role of H2O of over 35 kcal mol−1. Excellent agreement between 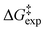 and DFT calculated Gibbs free activation energies,
and DFT calculated Gibbs free activation energies, 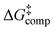 , was obtained with the use of three molecules of H2O for the neutral state of phloroglucinol (with the “in–in” configuration of the phenol OH groups) and taxifolin. In the ionic form of phloroglucinol, the mechanistic pathway with two molecules of H2O in the transition state (one of which involves the C
, was obtained with the use of three molecules of H2O for the neutral state of phloroglucinol (with the “in–in” configuration of the phenol OH groups) and taxifolin. In the ionic form of phloroglucinol, the mechanistic pathway with two molecules of H2O in the transition state (one of which involves the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O moiety) showed very good agreement with the experimental data. For the anionic form of taxifolin, the mechanistic pathway with three molecules of H2O in the transition state showed excellent agreement with the experimental
O moiety) showed very good agreement with the experimental data. For the anionic form of taxifolin, the mechanistic pathway with three molecules of H2O in the transition state showed excellent agreement with the experimental 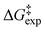 values. Among the various functionals used, the APFD/6-31+G(d) and B3LYP/6-31+G(d)/GD3BJ resulted in optimum agreement with
values. Among the various functionals used, the APFD/6-31+G(d) and B3LYP/6-31+G(d)/GD3BJ resulted in optimum agreement with 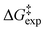 . The enthalpic term
. The enthalpic term 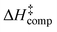 is considerably larger than the entropic term
is considerably larger than the entropic term 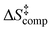 , in agreement with the experimental data. This indicates a dissociative mechanism of the loosely bound activated complex. The present results demonstrate the unique catalytic role of two and/or three molecules of H2O, through keto–enol tautomerization, with minor contribution of base-catalysis, in H/D exchange reactions in aromatic systems.
, in agreement with the experimental data. This indicates a dissociative mechanism of the loosely bound activated complex. The present results demonstrate the unique catalytic role of two and/or three molecules of H2O, through keto–enol tautomerization, with minor contribution of base-catalysis, in H/D exchange reactions in aromatic systems.



 Please wait while we load your content...
Please wait while we load your content...