Simulant chemistry for uranium and plutonium molten fuel salts: crystallographic investigation and thermodynamic modelling assessment of the NaCl–RECl3 and NaCl-MgCl2-RECl3 (RE = Ce, Nd) systems
Abstract
In this study, new insights into the solid state chemistry of the systems NaCl–RECl3 (RE = Ce, Nd) are presented, in which the intermediate compound suggested in the literature, i.e. Na3RE5Cl18, is investigated more closely. Our studies have revealed a solubility range around the intermediate composition in the form of the 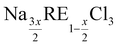 stoichiometry, and have allowed us to revisit the phase diagrams of the NaCl–RECl3 (RE = Ce, Nd) systems accordingly. Furthermore, we demonstrate that among the lanthanide chlorides, NdCl3 is the prime simulant candidate for the melting behaviour of PuCl3-based systems, while CeCl3 is most suited to simulate UCl3-based systems. This is corroborated in this work by comparing the melting profiles of the NaCl-MCl3, MgCl2-MCl3, and NaCl-MgCl2-MCl3 (M = Ce, Nd, U, Pu) systems. In doing so, the binary systems MgCl2-MCl3 (M = Ce, Nd) have been re-visited based on existing data in the literature and estimated mixing enthalpies. Extrapolations to the ternary systems NaCl-MgCl2-RECl3 (RE = Ce, Nd) have been made and compared to the available data in the literature, showing good agreement.
stoichiometry, and have allowed us to revisit the phase diagrams of the NaCl–RECl3 (RE = Ce, Nd) systems accordingly. Furthermore, we demonstrate that among the lanthanide chlorides, NdCl3 is the prime simulant candidate for the melting behaviour of PuCl3-based systems, while CeCl3 is most suited to simulate UCl3-based systems. This is corroborated in this work by comparing the melting profiles of the NaCl-MCl3, MgCl2-MCl3, and NaCl-MgCl2-MCl3 (M = Ce, Nd, U, Pu) systems. In doing so, the binary systems MgCl2-MCl3 (M = Ce, Nd) have been re-visited based on existing data in the literature and estimated mixing enthalpies. Extrapolations to the ternary systems NaCl-MgCl2-RECl3 (RE = Ce, Nd) have been made and compared to the available data in the literature, showing good agreement.



 Please wait while we load your content...
Please wait while we load your content...