The primary photolysis of aqueous acrylate
Abstract
We apply transient absorption spectroscopy supported by 2D-IR spectroscopy and density functional theory calculations to determine the primary photolysis of acrylate excited via the 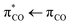 transition at 200 nm. Upon photoexcitation, about half of the excited acrylate anions return to the ground state and relax to equilibrium in 5 ps primarily through intermolecular coupling between the carboxylate group and the surrounding water. The rest of the excited acrylate anions dissociate. Three dissociation channels have been identified. In one reaction, decarboxylation of acrylate forms CO2 and CH2CH−. CH2CH− is protonated by water and forms ethene, C2H4, in <0.8 ps. In the second reaction, the excited acrylate anions dissociate to H2C
transition at 200 nm. Upon photoexcitation, about half of the excited acrylate anions return to the ground state and relax to equilibrium in 5 ps primarily through intermolecular coupling between the carboxylate group and the surrounding water. The rest of the excited acrylate anions dissociate. Three dissociation channels have been identified. In one reaction, decarboxylation of acrylate forms CO2 and CH2CH−. CH2CH− is protonated by water and forms ethene, C2H4, in <0.8 ps. In the second reaction, the excited acrylate anions dissociate to H2C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHO− and CO. In about 20 ps, H2C
CHO− and CO. In about 20 ps, H2C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHO− picks up a proton from water to produce vinyl alcohol, H2C
CHO− picks up a proton from water to produce vinyl alcohol, H2C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHOH. A third dissociation channel forms H2C
CHOH. A third dissociation channel forms H2C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHO˙ and CO−. H2C
CHO˙ and CO−. H2C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHO˙ abstracts a hydrogen atom from water and forms vinyl alcohol. Vinyl alcohol will tautomerize to acetaldehyde, but this occurs on a time scale longer than the experimental observation time of 0.56 ns.
CHO˙ abstracts a hydrogen atom from water and forms vinyl alcohol. Vinyl alcohol will tautomerize to acetaldehyde, but this occurs on a time scale longer than the experimental observation time of 0.56 ns.



 Please wait while we load your content...
Please wait while we load your content...