Abstract
Hyperconjugative interactions are widely recognized as stabilizing electronic effects in molecular systems. While their involvement in chemical transformations has been suggested, it remains as an open question whether their influence pertains to thermodynamic stabilization or if they directly affect intrinsic kinetic parameters (also referred to as the intrinsic activation barrier). In this work, we address a fundamental question: can 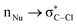 hyperconjugation directly shape the intrinsic barrier in an asymmetric SN2 reaction? To explore this, we present a systematic computational study using the MP2-SMD(THF)/cc-pVTZ level of theory, evaluating how
hyperconjugation directly shape the intrinsic barrier in an asymmetric SN2 reaction? To explore this, we present a systematic computational study using the MP2-SMD(THF)/cc-pVTZ level of theory, evaluating how 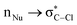 interactions influence the intrinsic activation barrier in reactions of the type Y− + CH3–Cl. It is worth noting that energy differences were also evaluated using the CCSD(T)-SMD(THF) method. Intrinsic barriers were extracted from the theoretical activation barrier using Marcus' theory to isolate the required energy for the nuclear reorganization free from thermodynamic bias. The donor–acceptor interactions were quantified through natural bond orbital (NBO) analysis; moreover, quantum theory of atoms in molecules (QTAIM) descriptors provided a complementary, orbital-independent perspective. In the studied systems, a strong correlation between the
interactions influence the intrinsic activation barrier in reactions of the type Y− + CH3–Cl. It is worth noting that energy differences were also evaluated using the CCSD(T)-SMD(THF) method. Intrinsic barriers were extracted from the theoretical activation barrier using Marcus' theory to isolate the required energy for the nuclear reorganization free from thermodynamic bias. The donor–acceptor interactions were quantified through natural bond orbital (NBO) analysis; moreover, quantum theory of atoms in molecules (QTAIM) descriptors provided a complementary, orbital-independent perspective. In the studied systems, a strong correlation between the 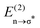 stabilization energies and the intrinsic activation barriers was observed, one that is not evident when considering apparent barriers. This distinction underscores, within the present framework, the importance of isolating intrinsic contributions when evaluating fundamental reactivity trends. QTAIM descriptors, such as the electron density at the bond critical point (ρBCP) and the |VBCP|/GBCP ratio, captured aspects of the local electronic environment, particularly electronegativity and polarizability, that were consistent with the intrinsic reactivity observed across the specific nucleophile families examined. In systems bearing α-substituents, the presence of secondary stabilizing interactions, likely involving non-covalent contacts between the nucleophile and C–H bonds of the electrophile, may contribute to lowering the intrinsic barrier. Together, these findings demonstrate that both NBO and QTAIM analyses are robust tools to determine the electronic contributions that govern reactivity and selectivity in the analyzed SN2 reactions. Furthermore, they position hyperconjugation not merely as a passive stabilizing effect but also as an active modulator capable of tuning intrinsic reactivity in organic reactions.
stabilization energies and the intrinsic activation barriers was observed, one that is not evident when considering apparent barriers. This distinction underscores, within the present framework, the importance of isolating intrinsic contributions when evaluating fundamental reactivity trends. QTAIM descriptors, such as the electron density at the bond critical point (ρBCP) and the |VBCP|/GBCP ratio, captured aspects of the local electronic environment, particularly electronegativity and polarizability, that were consistent with the intrinsic reactivity observed across the specific nucleophile families examined. In systems bearing α-substituents, the presence of secondary stabilizing interactions, likely involving non-covalent contacts between the nucleophile and C–H bonds of the electrophile, may contribute to lowering the intrinsic barrier. Together, these findings demonstrate that both NBO and QTAIM analyses are robust tools to determine the electronic contributions that govern reactivity and selectivity in the analyzed SN2 reactions. Furthermore, they position hyperconjugation not merely as a passive stabilizing effect but also as an active modulator capable of tuning intrinsic reactivity in organic reactions.

- This article is part of the themed collection: Celebrating Latin American Chemistry



 Please wait while we load your content...
Please wait while we load your content...
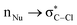 hyperconjugation interaction affects intrinsic reactivity in an SN2 reaction
hyperconjugation interaction affects intrinsic reactivity in an SN2 reaction