Abstract
The rate coefficient for the reaction of hydrogen sulfide (H2S) with hydroxyl radicals (OH), which plays an important role in producing sulfuric acid and sulfates in the Earth's atmosphere, was computed by combining the high-accuracy coupled-cluster HEAT-345Q(P) method for energetics, W. H. Miller's semi-classical transition state theory (SCTST) and two-dimensional E,J-resolved master equation analysis (2DME) for kinetics. The title reaction proceeds by H-abstraction through a well-skipping mechanism directly yielding products H2O + SH. Tunneling effects are found to be important at the extremely low temperatures of the interstellar medium, but insignificant above 300 K. The rate coefficient k1(T, P), calculated over the temperature range T = 10–2500 K and a wide pressure range, is found to be effectively independent of pressure for T = 200–2500 K, in which range k1(T) can be represented by 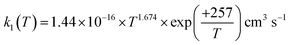 . The results agree within 30% with the experimental data available in the T range of 230 to 550 K. For the range of 200–500 K of atmospheric interest, we recommend the rate coefficient expression:
. The results agree within 30% with the experimental data available in the T range of 230 to 550 K. For the range of 200–500 K of atmospheric interest, we recommend the rate coefficient expression: 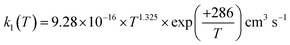 , based on the ab initio results of this work and the available experimental determinations.
, based on the ab initio results of this work and the available experimental determinations.



 Please wait while we load your content...
Please wait while we load your content...